|
Thermal desorption of H2O/CH3OH/CO ices
After investigating two component ice systems such as H2O/CO ices, we have now moved on to studying the thermal processing of the three component H2O/CH3OH/CO ice system using temperature programmed desorption (TPD).
TPD experiments were carried out at a range of water/methanol ratios. The ice was deposited as a 50 L exposure of the water/methanol mixture followed by a 5 L exposure of carbon monoxide. All of the ices were deposited at approximately 10 K. The traces show a CO desorption peak at 30 K (as seen in Figure 1) as well as peaks at about 80 K and 110 K (shown in the inset in Figure 1).
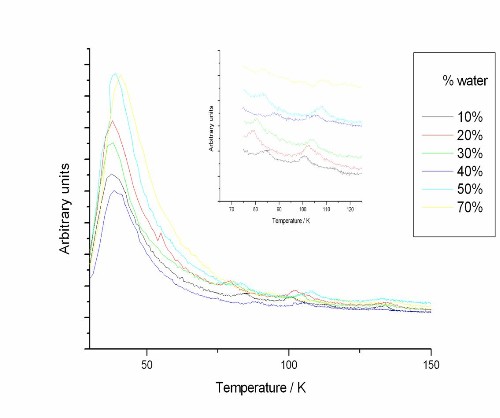
Figure 1. TPD traces for desorption of a CO overlayer from a water/methanol ice layer for a range of water/methanol ratios. Inset - Close up of the 75 K to 125 K range of the TPD traces
The broad peak centred at about 30K is consistent with desorption of the CO multilayer on top of the water/methanol ice. The presence of the two higher temperature peaks suggests that some of the CO remains in the water/methanol ice after, trapped in pores in the ice.
Figures 2 and 3 show desorption traces for water and methanol. Despite dosing the water and methanol as a mixture, the two species desorb at different temperatures with no co-desorption, indicating that phase separation occurs as the ice is heated.
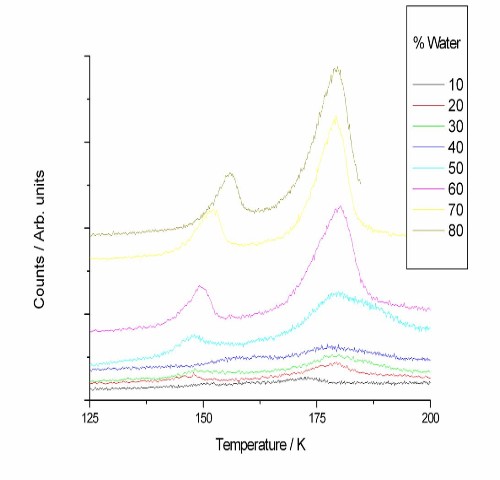
Figure 2. TPD traces for desorption of water from a water/methanol ice layer under a layer of CO for a range of water/methanol ratios.
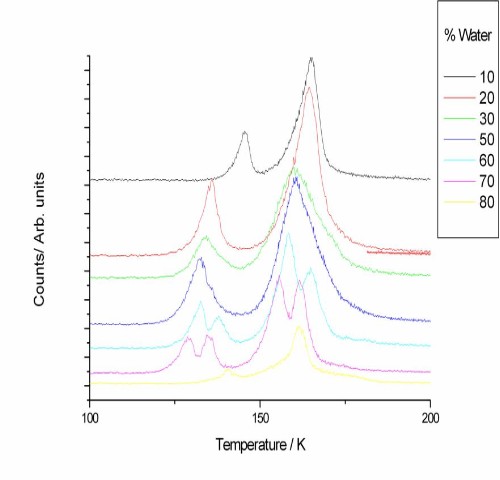
Figure 3. Figure 3. TPD traces for desorption of methanol from a water/methanol ice layer under a layer of CO for a range of water/methanol ratios.
The origin of the double peak structure seen in some of the methanol desorption traces is currently unknown and requires further investigation.
|
|



