The Heriot-Watt Astrochemistry Research Group |
 |
Photon stimulated desorption of hot molecules from interstellar ices

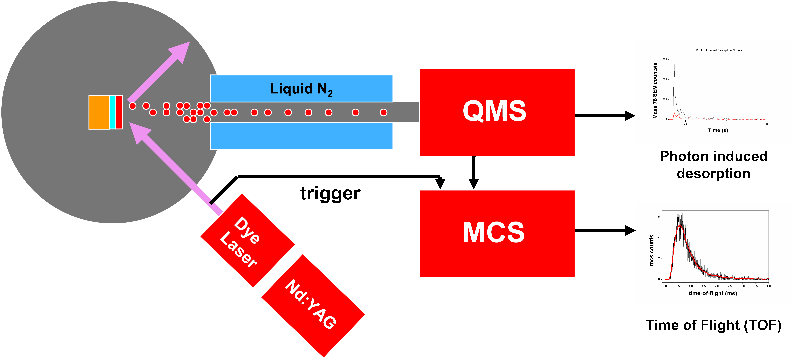
In these experiments, a sapphire substrate was used in order to facilitate cooling to cryogenic temperatures (80-100 K), whilst eliminating and metal-mediated effects that would not be relevant in an interstellar environment. The water and benzene layered ices were produced by backfilling the chamber to 10-7 mbar for 500s, a dose of 200 L. Such a dose however produce a much thinner film on benzene than water due to the differences in sticking probabilities and ion gauage sensitivities. The systems are illustrated in Figure 3. Following adsorption the ices were irradiated with photons at 2 pulse energies of 1.1 mJ and 1.8 mJ. Thre wavelengths were used, 250 nm is on-resonance with a vibronic transition in the benzene 1B2u <-- 1A1g , whilst 248.8 nm (near-resonance) is in a minimum. 275 nm was used as an off-resonance reference. The irradation time was 60 s with a pulse length of ~10 ns and a frequency of 10 Hz. Desorbed benzene and water was detected by monitoring the 78 amu and 18 amu mass channels respectively. TOF profiles were averaged over 30 spots on the sample surface.
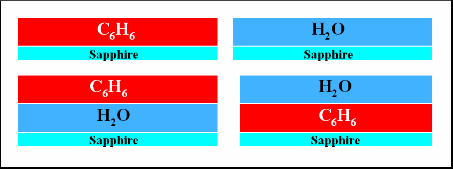
Figure 4 shows ToF profiles for benzene and water following irradiation with UV photons on resonance with the benzene absorption (250.0 nm). The left hand panel demonstrates the effect of layer configuration. It is clear that when a cap of water is present over the benzene layer, the desorption of benzene is inhibited. A small amount of benzene is observed to desorb when benzene is not present, indicating that a substrate mediated desorption channel involving absorption by the sapphire is also in operation. The amount of water desorbed is increased when benzene is present, which suggests an energy transfer mechanism between the benzene and the water. The right hand panel illustrates the dependence of the observed desorptions on laser pulse energy. The amount of both benzene and water desorbed increases broadly in proportion with the laser pulse energy. This is indicates that single photon processes are responsible for the desorption of both benzene and water. This is important, as the effect of multiphoton processes in the ISM is negligable due to the low photon flux.
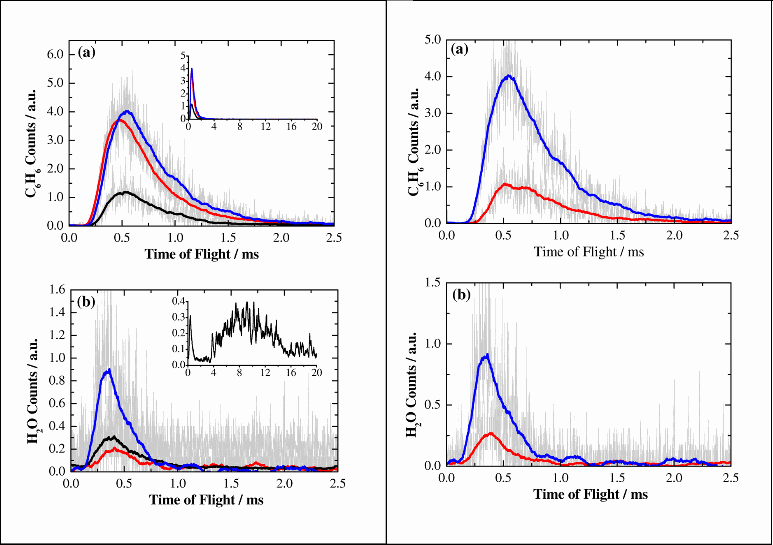
Figure 4. (Right Hand Panel): ToF profiles for (a) C6H6 and (b)H2O (wavelength=250.0 nm). Desorption is from the Al2O3/H2O/C6H6 system. Red (blue) lines correspond to a laser pulse energy of 1.1 mJ (1.8 mJ)
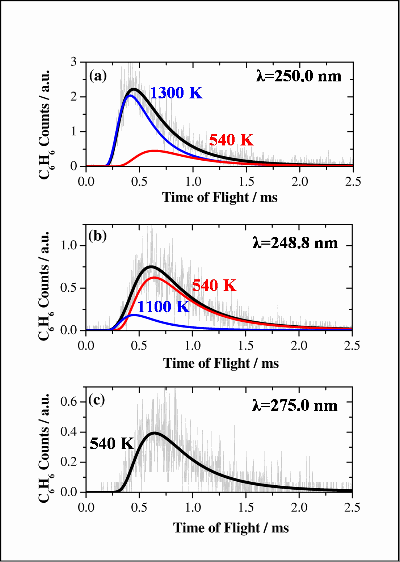
Figure 5 shows 4th order Maxwell-Boltzmann fits to the benzene ToF profiles. The profiles are compared for the three wavelengths used in these experiments. The slow component observed off-resonance (c) is attributed to substrate mediated desorption. This component has been fixed at 540 K for fitting at the other two wavelengths. The two component fitting for C6H6 at (a) 250.0 nm shows a much more intense, faster component with a temperature of around 1300 K. This can be attributed to desorption following resonant absorption by the C6H6 molecules. At the near-resonance wavelength (b) 248.8 nm desorption appears to be dominated by the substrate mediated component, with a small, fast resonant desorption component. For both components the molecular temperatures are greatly elevated with respect to the substrate temperature – i.e. desorption of hot molecules. The desorption of H2O can also be fitted with distributions having T~400 K, also indicating the desorption of hot molecules.
The desorption characteristics are likely related to the morphology of the ice, and future experiments will aim to study more realistic ice mixtures. A fuller investigation of the power dependence is also required in order to be certain of the absence of multiphoton processes. Alternative substrates will also be investigated to eliminate the substrate mediated desorption channel. It will also be important to extend this work to the study of PAHs themselves and it is anticipated that a study of electron stimulated processes in these systems will be conducted.